Vero Cell Vaccine Efficacy
KATHMANDU JUNE 25. Production of cell-based vaccines.
Vaccines Free Full Text Development And Evaluation Of Vero Cell Derived Master Donor Viruses For Influenza Pandemic Preparedness Html
It is the also first vaccine that will carry a vaccine vial monitor a small sticker on the vaccine vials that change color as the vaccine is exposed to heat letting health.

Vero cell vaccine efficacy. Additionally the Vero cell. Vero E6 cells were cultured in T25 flasks to near confluency. To produce viral vaccines candidate vaccine viruses are grown in mammalian tissue culture of cells with a finite lifespan.
Efficacy Safety and Immunogenicity of Inactivated SARS-CoV-2 Vaccines Vero Cell to Prevent COVID-19 in Healthy Adult Population In Peru Healthy Adult Population In Peru Covid-Peru The safety and scientific validity of this study is the responsibility of the study sponsor and investigators. Vaccine efficacy is measured in RCTs usually under optimal conditions where vaccine storage and delivery are monitored and participants are usually in good health or selected for a specific health status. Akova M Unal S.
30 Sinopharm announces that the vaccine has an efficacy of 7934 percent leading the Chinese government to approve itThe company has yet to publish detailed results of their Phase 3. Earlier experience of inactivated vaccine manufacturing and the availability of a BSL-3 facility equipped with a well-characterised Vero cell manufacturing platform aided us in the rapid. What vaccine is being registered.
The serum-virus mixtures were inoculated into wells of a 96-well microplate with preformed Vero E6 ATCC CRL-1586 TM cell monolayers and adsorbed at 37 C with 5 CO 2 for 05 h. Page 1 of 10 FREQUENTLY ASKED QUESTIONS FAQ ABOUT CORONAVAC VACCINE Q1. A randomized double-blind placebo-controlled phase III clinical trial to evaluate the efficacy and safety of SARS-CoV-2 vaccine inactivated Vero cell.
The candidate vaccine virus strain will replicate. Its easy storage requirements make it highly suitable for low-resource settings. A total of 100 μL supernatant from infected flasks were added and gently mixed.
After a 1-h incubation at 37 C5 CO 2 inoculum was discarded and fresh complete medium was added. COVID-19 Vaccine Vero cell Inactivated. The third phase clinical trials of the vaccine took place in Malaysia in January this year.
Efficacy safety and immunogenicity of a Vero-cell-culture-derived trivalent influenza. Vaccine Efficacy 10000 CI 95 2033-10000 Efficacy against symptomatic COVID-19 cases in Indonesia Efficacy after 14 days with two doses of vaccination Data as of January 11 2021. The Sinopharm product is an inactivated vaccine called SARS-CoV-2 Vaccine Vero Cell.
In October 2014 Sanofi Pasteur and Immune Design announced a collaboration. Prior to infection spent cell culture medium was replaced with 2 mL fresh DMEM without FBS. Nepal has granted emergency approval to the Chinese Vero Cell vaccine given the active caseload that stands at 277944 on Monday while 273240 people have recovered in Nepal.
The Sanofi Pasteur vaccine candidate HSV529 is derived from HSV-2 strain 186 produced in Vero cells expressing HSV2 UL5 and UL29. The Drug Control Authority DCA in its 354th meeting has granted CoronaVac Suspension for Injection SARS-CoV-2 Vaccine Vero Cell Inactivated Product Registration Holder. Vaccine and the study.
Covaxin the countrys first indigenous Covid-19 vaccine was developed with seed strains received from the National Institute of Virology NIV using Whole Virion Inactivated Vero Cell derived platform technology. Barrett PN Berezuk G Fritsch S et al. The World Health Organization WHO has refuted the claims that a mix and match regimen of Vero Cell and AstraZeneca.
The move came one month after WHO listed Vero Cell vaccine for emergency use earlier in May the first non-Western vaccine to do so which enables the vaccine to be used in the supply for the global vaccine sharing initiative COVAX and helps poor and developing countries access COVID-19 vaccines as well as allows countries to expedite their own regulatory approval to import and. These cells are typically Madin-Darby Canine Kidney cells but others are also used including monkey cell lines pMK and Vero and human cell lines HEK 293 MRC 5 PerC6 PMK and WI-38. S1 1527 the overall.
A structured summary of a study protocol for a randomised controlled trial. These interim recommendations efer tor the inactivated COVID-19 vaccine Vero cell manufactured by the Beijing Institute of Biological Products Co Limited BIBP a subsidiary of the China National Biotec Group CNBG. Sanofi Pasteur contributed HSV529 a clinical-stage replication-defective HSV vaccine product candidate and Immune Design will.
The China National Pharmaceutical Group corporation Sinopharm is. Vero Cell is an inactivated vaccine like those of polio and influenza. Pharmaniaga Lifescience Sdn Bhd.
On Sunday Nepals Ministry of Health and Population made the announcement where it hinted that at least 400000 people will be initially vaccinated using the Chinese. In a post hoc analysis of vaccine efficacy at more than 14 days after a single injection through October 31 2020 as a proxy for infection by a nonB1351 variant Fig. The Vero cell line is the most used continuous cell line for viral vaccine manufacturing with more than 40 years of accumulated experience in the vaccine industry.
500 000 Doses Of Sinopharm Covid 19 Vaccine Arrive In Vietnam Today

Integrated Control Of Covid 19 In Resource Poor Countries International Journal Of Infectious Diseases

Who Approves China S Sinopharm Covid 19 Vaccine For Emergency Use Has 79 Efficacy Coronavirus Outbreak News

Questions And Answers On Sinopharm Covid 19 Vaccine Bangkok Hospital
Https Cdn Who Int Media Docs Default Source Immunization Sage 2021 April 1 Sage29apr2021 Sinopharm Pdf Sfvrsn Ddf0d841 5

Two Covid 19 Vaccines Approved In China In Less Than 24 Hours 2021 03 02 Bioworld
China S Vaccine Diplomacy Assumes Geopolitical Importance Merics

Coronavirus How Effective Are The Chinese Vaccines Science In Depth Reporting On Science And Technology Dw 01 02 2021
China S Covid Vaccine From Sinopharm Is 86 Effective Uae Says

Novavax News Articles Etc European Pharmaceutical Review
Https Cdn Who Int Media Docs Default Source Immunization Sage 2021 April 1 Sage29apr2021 Sinopharm Pdf Sfvrsn Ddf0d841 5
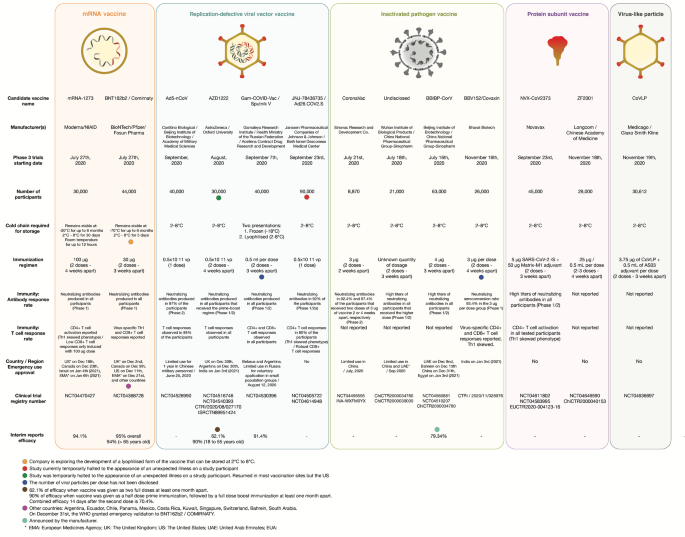
Sars Cov 2 Vaccines Strategies A Comprehensive Review Of Phase 3 Candidates Npj Vaccines

Coronavirus Confusion Over Efficacy Of Chinese Vaccines Science In Depth Reporting On Science And Technology Dw 13 04 2021
World Health Organization Who What S The Difference Between Covid 19 Vaccine Efficacy And Effectiveness Vaccine Efficacy Refers To How The Vaccine Performs In Ideal Conditions Controlled Clinical Trials Vaccine

Vero Cell Produced Vaccines Against Viral Diseases Download Table

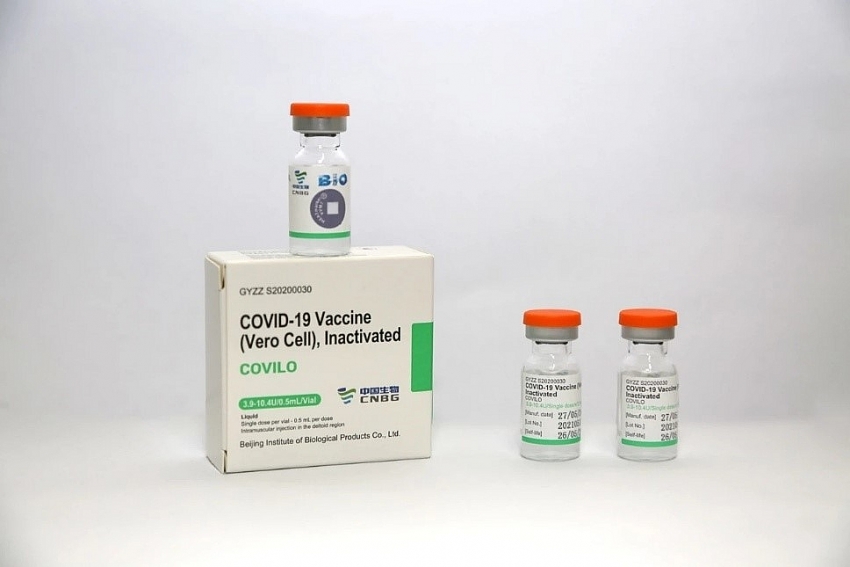
Sinopharm Vero Cell Inactivated Covid 19 Vaccine
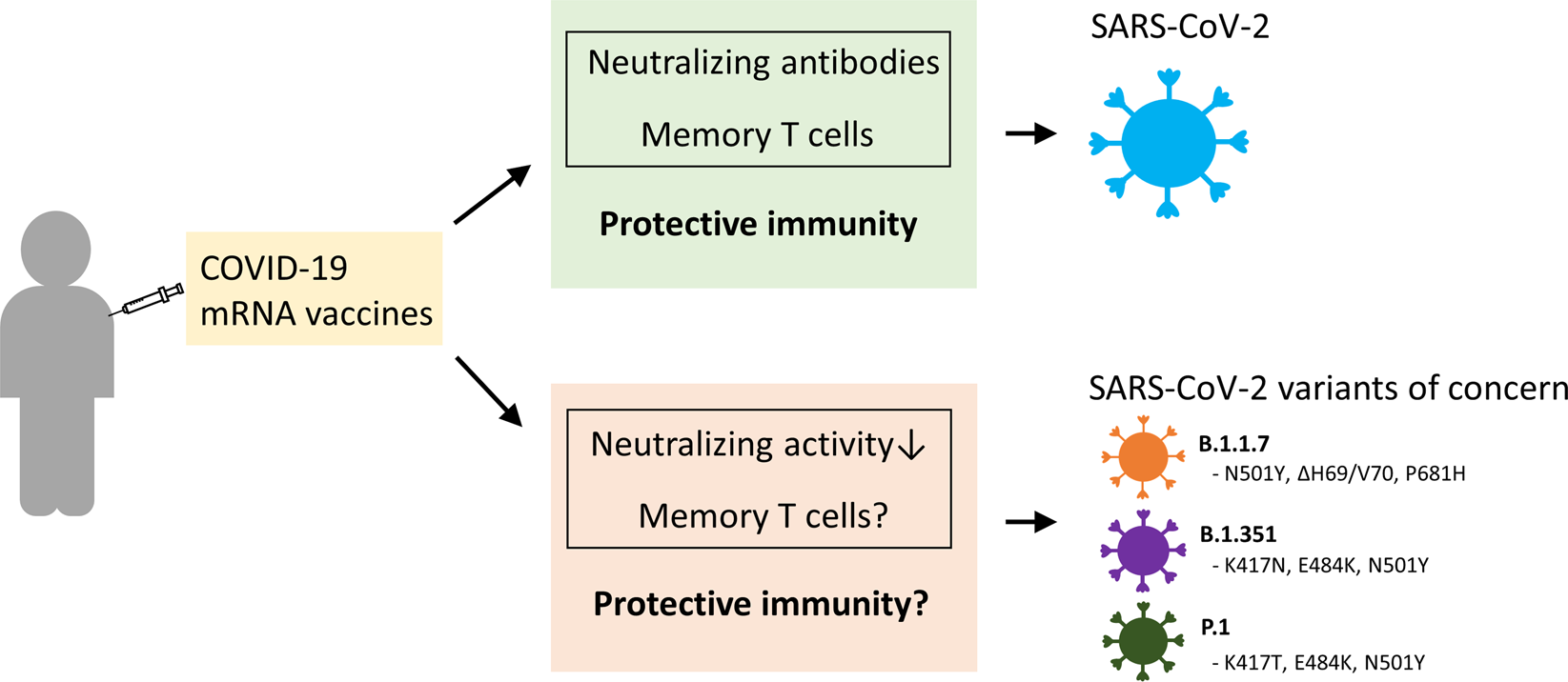
Sars Cov 2 Mutations Vaccines And Immunity Implication Of Variants Of Concern Signal Transduction And Targeted Therapy
Https Cdn Who Int Media Docs Default Source Immunization Sage 2021 April 1 Sage29apr2021 Sinopharm Pdf Sfvrsn Ddf0d841 5

China Vaccine Vero Cell Green Light For Human Trial In Bangladesh The Daily Star

Post a Comment for "Vero Cell Vaccine Efficacy"